Multiple Choice
Sodium hydroxide is added to a buffer solution of ammonia, N 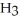 , and ammonium chloride,
, and ammonium chloride, 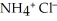 . What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
. What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
A) Sodium hydroxide reacts with both the ammonia and ammonium chloride, so the concentration of both decreases.
B) Sodium hydroxide reacts with only the ammonium chloride, so the concentration of the ammonium chloride decreases and the concentration of ammonia increases.
C) Sodium hydroxide reacts with both the ammonia and ammonium chloride, so the concentration of both increases.
D) Sodium hydroxide reacts with only the ammonia, so the concentration of the ammonium chloride increases and the concentration of ammonia decreases.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: According to the following reaction,which molecule is
Q21: What happens to the pH of soda
Q37: Which of the following statements about buffers
Q46: What is the main characteristic of a
Q85: Why are aqueous solutions of highly charged
Q119: Why do we use the pH scale
Q136: As the hydronium ion concentration increases,the pH<br>A)goes
Q137: Which of the following statements describes a
Q157: What would be the best explanation for
Q175: For the following acid-base reaction,identify what is