Multiple Choice
What is the hydroxide ion concentration in an aqueous solution where the pH = 5?
A) The hydronium ion concentration equals 1 × 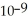 .
.
B) The hydronium ion concentration equals 1 × 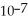 .
.
C) The hydronium ion concentration equals 1 × 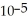 .
.
D) The hydronium ion concentration equals 1 × 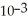 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Q45: When the hydronium ion concentration equals 1
Q46: What is the main characteristic of a
Q71: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q72: Sometimes an individual going through a traumatic
Q72: Cutting back on the pollutants that cause
Q78: Suggest why people once washed their hands
Q97: Which of the following statements is not
Q119: Why do we use the pH scale
Q136: As the hydronium ion concentration increases,the pH<br>A)goes
Q140: For the following reaction,identify whether the compound