Multiple Choice
Sometimes an individual going through a traumatic experience cannot stop hyperventilating. In such a circumstance, it is recommended that the individual breath into a paper bag or cupped hands as a useful way to avoid an increase in blood pH, which can cause the person to pass out. Explain how this works.
A) Carbon dioxide initially exhaled is reinhaled to help maintain adequate levels of carbonic acid in the blood.
B) It helps to slow their breathing down to prevent excess oxygen from entering the blood.
C) The paper absorbs C 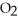 to prevent the accumulation of excess C
to prevent the accumulation of excess C 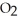 in the blood.
in the blood.
D) The paper bag helps to retain the water vapor leaving the mouth to prevent an increase in the concentration of lactic acid in the body.
Correct Answer:

Verified
Correct Answer:
Verified
Q45: When the hydronium ion concentration equals 1
Q57: Which of the following solutions is the
Q71: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q72: Cutting back on the pollutants that cause
Q74: What is the hydroxide ion concentration in
Q78: Suggest why people once washed their hands
Q97: Which of the following statements is not
Q136: As the hydronium ion concentration increases,the pH<br>A)goes
Q138: According to the following reaction,which molecule is
Q140: For the following reaction,identify whether the compound