Multiple Choice
The figure shows a pV diagram for 2.6 g of ideal helium gas that undergoes the process 1 → 2 → 3. Find the value of volume V3. The atomic mass of helium is 4.0 g/mol, and R = 8.31 J/mol ∙ K. 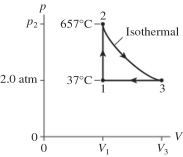
A) 25 L
B) 99 L
C) 50 L
D) 12 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: A refrigerator has an interior volume of
Q16: By what primary heat transfer mechanism does
Q27: At what temperature would the root-mean-square speed
Q27: Two metal rods,one silver and the other
Q64: A heat-conducting rod that is wrapped in
Q77: The volume coefficient of thermal expansion for
Q82: The rms speed of a certain sample
Q86: A mercury thermometer has a glass bulb
Q171: If you add 700 kJ of
Q173: The figure shows a pV diagram for