Multiple Choice
A 648-g empty iron kettle is put on a stove.How much heat,in joules,must it absorb to raise its temperature from 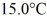 to
to 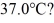 (The specific heat for iron is
(The specific heat for iron is 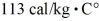 ,
, 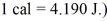
A) 6740 J
B) 11,300 J
C) 1610 J
D) 16,100 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: When a solid melts,<br>A) the temperature of
Q54: A machinist needs to remove a tight
Q55: A person makes ice tea by adding
Q56: A substance has a melting point of
Q57: The coefficient of volume expansion of olive
Q58: A rod,with sides insulated to prevent heat
Q59: A concrete wall of a cold storage
Q60: A solid concrete wall 4.0 m by
Q61: Under steady state conditions,a piece of wood
Q63: The walls of an ice chest are