Multiple Choice
A substance has a melting point of 20°C and a heat of fusion of 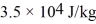 .The boiling point is 150°C and the heat of vaporization is
.The boiling point is 150°C and the heat of vaporization is 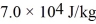 at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are
at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 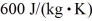 ,
, 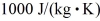 ,and
,and 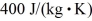 ,respectively.The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C,at a pressure of 1.0 atmosphere,is closest to
,respectively.The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C,at a pressure of 1.0 atmosphere,is closest to
A) 70 kJ.
B) 14 kJ.
C) 21 kJ.
D) 30 kJ.
E) 44 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Q51: A 200-g metal container,insulated on the outside,holds
Q52: A 905-g meteor impacts the earth at
Q53: When a solid melts,<br>A) the temperature of
Q54: A machinist needs to remove a tight
Q55: A person makes ice tea by adding
Q57: The coefficient of volume expansion of olive
Q58: A rod,with sides insulated to prevent heat
Q59: A concrete wall of a cold storage
Q60: A solid concrete wall 4.0 m by
Q61: Under steady state conditions,a piece of wood