Multiple Choice
A 200-g metal container,insulated on the outside,holds 100 g of water in thermal equilibrium at 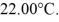 A 21-g ice cube,at the melting point,is dropped into the water,and when thermal equilibrium is reached the temperature is 15.00°C.Assume there is no heat exchange with the surroundings.For water,the specific heat is 4190 J/kg • K and the heat of fusion is
A 21-g ice cube,at the melting point,is dropped into the water,and when thermal equilibrium is reached the temperature is 15.00°C.Assume there is no heat exchange with the surroundings.For water,the specific heat is 4190 J/kg • K and the heat of fusion is 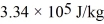 .The specific heat for the metal is closest to
.The specific heat for the metal is closest to
A) 3850 J/kg • K.
B) 2730 J/kg • K.
C) 4450 J/kg • K.
D) 4950 J/kg • K.
E) 5450 J/kg • K.
Correct Answer:

Verified
Correct Answer:
Verified
Q46: When a vapor condenses,<br>A) the temperature of
Q47: Two metal rods,one silver and the other
Q48: Suppose that a steel bridge,1000 m long,was
Q49: It is necessary to determine the specific
Q50: The exterior of a supersonic airplane is
Q52: A 905-g meteor impacts the earth at
Q53: When a solid melts,<br>A) the temperature of
Q54: A machinist needs to remove a tight
Q55: A person makes ice tea by adding
Q56: A substance has a melting point of