Multiple Choice
If you add 700 kJ of heat to 700 g of water at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 2.26 × 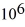 J/kg and its specific heat is is
J/kg and its specific heat is is 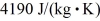 .
.
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A substance has a melting point of
Q2: The hole for a bolt in a
Q3: A heat conducting rod,1.40 m long,is made
Q4: Betelgeuse is a red supergiant star in
Q6: An architect is interested in estimating the
Q7: A rod has a length 2.00000 m
Q8: 1)000 L of water at 20.00°C will
Q9: A radiating body originally has a Kelvin
Q10: (a)Internal human body temperature is often stated
Q11: A 400-g piece of metal at 120.0°C