Multiple Choice
A substance has a melting point of 20°C and a heat of fusion of 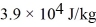 .The boiling point is
.The boiling point is 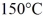 and the heat of vaporization is
and the heat of vaporization is 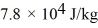 at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg • K) ,1000 J/(kg • K) ,and 400 J/(kg • K) ,respectively.The quantity of heat required to raise the temperature of
at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg • K) ,1000 J/(kg • K) ,and 400 J/(kg • K) ,respectively.The quantity of heat required to raise the temperature of 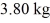 of the substance from
of the substance from 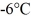 to
to 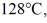 at a pressure of 1.0 atm,is closest to
at a pressure of 1.0 atm,is closest to
A) 620 kJ.
B) 470 kJ.
C) 560 kJ.
D) 210 kJ.
E) 770 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The hole for a bolt in a
Q3: A heat conducting rod,1.40 m long,is made
Q4: Betelgeuse is a red supergiant star in
Q5: If you add 700 kJ of heat
Q6: An architect is interested in estimating the
Q7: A rod has a length 2.00000 m
Q8: 1)000 L of water at 20.00°C will
Q9: A radiating body originally has a Kelvin
Q10: (a)Internal human body temperature is often stated
Q11: A 400-g piece of metal at 120.0°C