Multiple Choice
If we double the root-mean-square speed (thermal speed) of the molecules of a gas,then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of 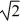 .
.
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: The root-mean-square speed (thermal speed)of the molecules
Q33: At 50.0°C,the average translational kinetic energy of
Q34: A cold trap is set up to
Q35: A 5.0-liter gas tank holds 1.7 moles
Q36: A vertical tube that is closed at
Q38: What is the mean free path of
Q39: Sometimes an experiment requires a certain pure
Q40: What is the average kinetic energy of
Q41: A container is filled with a mixture
Q42: The figure shows a 50-kg frictionless cylindrical