Multiple Choice
Sometimes an experiment requires a certain pure gas to be used at reduced pressure.One way to achieve this is to purchase a sealed glass container filled with the gas,and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet.If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 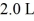 ,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
A) 33 kPa
B) 50 kPa
C) 300 kPa
D) 200 kPa
Correct Answer:

Verified
Correct Answer:
Verified
Q34: A cold trap is set up to
Q35: A 5.0-liter gas tank holds 1.7 moles
Q36: A vertical tube that is closed at
Q37: If we double the root-mean-square speed (thermal
Q38: What is the mean free path of
Q40: What is the average kinetic energy of
Q41: A container is filled with a mixture
Q42: The figure shows a 50-kg frictionless cylindrical
Q43: A sealed 89- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed
Q44: A sealed container holds 0.020 moles of