Multiple Choice
A 0.10- 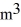 gas tank holds 5.0 moles of nitrogen gas (N2) ,at a temperature of
gas tank holds 5.0 moles of nitrogen gas (N2) ,at a temperature of 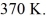 The atomic mass of nitrogen is 14 g/mol,the molecular radius is
The atomic mass of nitrogen is 14 g/mol,the molecular radius is 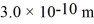 ,and the Boltzmann constant is
,and the Boltzmann constant is 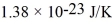 .The root-mean-square speed (thermal speed) of the molecules is closest to
.The root-mean-square speed (thermal speed) of the molecules is closest to
A) 570 m/s.
B) 810 m/s.
C) 410 m/s.
D) 22 m/s.
E) 99 m/s.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: A sealed 26- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed
Q26: A 25-L container holds ideal hydrogen (H<sub>2</sub>)gas
Q27: A sample of an ideal gas is
Q28: The number of molecules in one mole
Q29: Dust particles are pulverized rock,which has density
Q31: A sealed container holds 0.020 moles of
Q32: The root-mean-square speed (thermal speed)of the molecules
Q33: At 50.0°C,the average translational kinetic energy of
Q34: A cold trap is set up to
Q35: A 5.0-liter gas tank holds 1.7 moles