Multiple Choice
A 25-L container holds ideal hydrogen (H2) gas at a gauge pressure of 0.25 atm and a temperature of 0°C.What mass of hydrogen gas is in this container? The ATOMIC mass of hydrogen is 1.0 g/mol,the ideal gas constant is 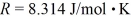 =
= 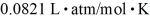 ,and
,and 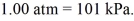
A) 1.4 g
B) 2.8 g
C) 4.2 g
D) 5.6 g
E) 6.3 g
Correct Answer:

Verified
Correct Answer:
Verified
Q21: An ideal gas is kept in a
Q22: A fixed amount of ideal gas is
Q23: The mean free path of an oxygen
Q24: What is the mass density of argon
Q25: A sealed 26- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed
Q27: A sample of an ideal gas is
Q28: The number of molecules in one mole
Q29: Dust particles are pulverized rock,which has density
Q30: A 0.10- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A 0.10-
Q31: A sealed container holds 0.020 moles of