Multiple Choice
What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 300 K? The Boltzmann constant is 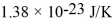 .
.
A) 1.7 × 10-21 J
B) 8.3 × 10-21 J
C) 6.2 × 10-21 J
D) 2.1 × 10-21 J
E) 4.1 × 10-21 J
Correct Answer:

Verified
Correct Answer:
Verified
Q41: A container is filled with a mixture
Q42: The figure shows a 50-kg frictionless cylindrical
Q43: A sealed 89- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed
Q44: A sealed container holds 0.020 moles of
Q45: Assuming the radius of diatomic molecules is
Q47: A mole of oxygen (O<sub>2</sub>)molecules and a
Q48: If the temperature of a fixed amount
Q49: The average molecular kinetic energy of a
Q50: The root-mean-square speed (thermal speed)of a certain
Q51: A weather balloon contains 12.0 m<sup>3</sup> of