Multiple Choice
A sealed 89- 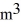 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 350 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is
tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 350 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is 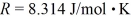 =
= 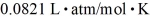 .The initial pressure of the gas is closest to
.The initial pressure of the gas is closest to
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.
Correct Answer:

Verified
Correct Answer:
Verified
Q38: What is the mean free path of
Q39: Sometimes an experiment requires a certain pure
Q40: What is the average kinetic energy of
Q41: A container is filled with a mixture
Q42: The figure shows a 50-kg frictionless cylindrical
Q44: A sealed container holds 0.020 moles of
Q45: Assuming the radius of diatomic molecules is
Q46: What is the average translational kinetic energy
Q47: A mole of oxygen (O<sub>2</sub>)molecules and a
Q48: If the temperature of a fixed amount