Multiple Choice
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 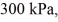 the initial volume is
the initial volume is 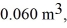 and the initial temperature is
and the initial temperature is 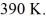 The ideal gas constant is
The ideal gas constant is 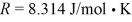 .The final pressure is
.The final pressure is 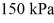 and the final temperature is
and the final temperature is 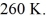 The work done by the gas is closest to
The work done by the gas is closest to
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.
Correct Answer:

Verified
Correct Answer:
Verified
Q34: An ideal gas is compressed in a
Q35: The temperature of an ideal gas in
Q36: In an isochoric process,the internal (thermal)energy of
Q37: The figure shows a pV diagram for
Q38: The pV diagram shown is for 7.50
Q40: The process shown in the pV diagram
Q41: A quantity of ideal gas requires 800
Q42: An ideal gas initially at 300 K
Q43: During an adiabatic process,an ideal gas does
Q44: An ideal monatomic gas cools from 455.0