Short Answer
An ideal Carnot engine operates between reservoirs having temperatures of 125°C and
-20°C.Each cycle the heat expelled by this engine is used to melt 30.0 g of ice at 0.00°C.The heat of fusion of water is 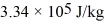 and the heat of vaporization of water is
and the heat of vaporization of water is 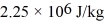 .
.
(a)How much work does this engine do each cycle?
(b)How much heat per cycle does this engine absorb at the hot reservoir?
Correct Answer:

Verified
(a)5740 J ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q40: A heat engine with an efficiency of
Q41: A system consists of two very large
Q42: A refrigerator has a coefficient of performance
Q43: The entropy of an isolated system must
Q44: An engine manufacturer makes the claim that
Q45: A perfect Carnot engine operates between the
Q46: A Carnot engine operates between a high
Q47: The graph in the figure shows a
Q49: A nuclear fission power plant has an
Q50: What is the change in entropy of