Multiple Choice
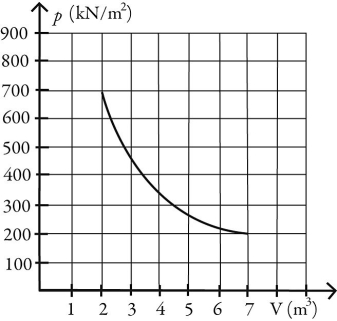
What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is  .
. 
A) 221 J/K
B) 104 J/K
C) 63.1 J/K
D) 45.2 J/K
E) 90.8 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: A heat engine with an efficiency of
Q41: A system consists of two very large
Q42: A refrigerator has a coefficient of performance
Q43: The entropy of an isolated system must
Q44: An engine manufacturer makes the claim that
Q45: A perfect Carnot engine operates between the
Q46: A Carnot engine operates between a high
Q47: The graph in the figure shows a
Q48: An ideal Carnot engine operates between reservoirs
Q49: A nuclear fission power plant has an