Multiple Choice
Gamma rays are photons with very high energy.How many visible-light photons with a wavelength of 500 nm would you need to match the energy of a gamma-ray photon with energy 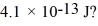 (h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)
(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)
A) 1.0 × 106
B) 1.4 × 108
C) 6.2 × 109
D) 3.9 × 103
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: A photon of wavelength 29 pm is
Q18: The energy of an electron state has
Q19: If the accuracy in measuring the position
Q20: Upon being struck by 240-nm photons,a metal
Q21: A nonrelativistic proton is confined to a
Q23: A stopping potential of 0.50 V is
Q24: An electron inside a hydrogen atom is
Q25: In a photoelectric effect experiment,electrons emerge from
Q26: A metal having a work function of
Q27: A 440-nm spectral line is produced by