Multiple Choice
An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? ( h = 1.055 × 10-34 J • s, 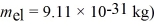
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: If the accuracy in measuring the position
Q20: Upon being struck by 240-nm photons,a metal
Q21: A nonrelativistic proton is confined to a
Q22: Gamma rays are photons with very high
Q23: A stopping potential of 0.50 V is
Q25: In a photoelectric effect experiment,electrons emerge from
Q26: A metal having a work function of
Q27: A 440-nm spectral line is produced by
Q28: When a certain metal is illuminated by
Q29: When a metal surface is illuminated with