Multiple Choice
Light shines through atomic hydrogen gas.It is seen that the gas absorbs light readily at a wavelength of 91.63 nm.What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level.(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s, 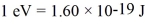 ,the Rydberg constant is R = 1.097 × 107 m-1)
,the Rydberg constant is R = 1.097 × 107 m-1)
A) 14
B) 16
C) 11
D) 21
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The energy of the ground state in
Q2: A nonrelativistic electron is accelerated from rest
Q3: How fast must a nonrelativistic electron move
Q4: A molecule of roughly spherical shape has
Q5: Calculate the kinetic energy (in eV)of a
Q5: An unstable particle produced in a high-energy
Q7: A nonrelativistic proton is confined to a
Q9: The wavelength of a ruby laser is
Q10: The energy of an electron state has
Q11: The Bohr radius of the hydrogen atom