Multiple Choice
A molecule of roughly spherical shape has a mass of 6.10 × 10-25 kg and a diameter of 0.70 nm.The uncertainty in the measured position of the molecule is equal to the molecular diameter.What is the minimum uncertainty in the speed of this molecule? 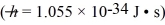
A) 0.12 m/s
B) 1.2 m/s
C) 12 m/s
D) 0.012 m/s
E) 0.0012 m/s
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The energy of the ground state in
Q2: A nonrelativistic electron is accelerated from rest
Q3: How fast must a nonrelativistic electron move
Q5: Calculate the kinetic energy (in eV)of a
Q5: An unstable particle produced in a high-energy
Q6: Light shines through atomic hydrogen gas.It is
Q7: A nonrelativistic proton is confined to a
Q9: The wavelength of a ruby laser is
Q10: The energy of an electron state has
Q11: The Bohr radius of the hydrogen atom