Multiple Choice
A hydrogen atom initially in the n = 6 state decays to the n = 2 state.The emitted photon is detected in a photographic plate.What is the wavelength of the detected photon? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J, 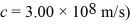
A) 410 nm
B) 93.8 nm
C) 1090 nm
D) 93.1 nm
Correct Answer:

Verified
Correct Answer:
Verified
Q17: An electron inside a hydrogen atom is
Q18: A hydrogen atom makes a downward transition
Q19: An electric current through a tungsten filament
Q20: In a ruby laser,an electron jumps from
Q21: How many photons per second emerge from
Q24: A perfectly black body at 100°C emits
Q25: A certain particle's energy is measured by
Q26: The energy of the ground state in
Q27: An ultraviolet source produces a monochromatic beam
Q37: If the accuracy in measuring the velocity