Multiple Choice
An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? ( h = 1.055 × 10-34 J • s, 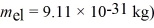
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Light excites atomic hydrogen from its lowest
Q14: A gas of helium atoms (each of
Q15: An electric current through a tungsten filament
Q16: In a double slit experiment,a beam of
Q18: A hydrogen atom makes a downward transition
Q19: If the accuracy in measuring the position
Q19: An electric current through a tungsten filament
Q20: In a ruby laser,an electron jumps from
Q21: How many photons per second emerge from
Q22: A hydrogen atom initially in the n