Multiple Choice
A gas of helium atoms (each of mass 6.65 × 10-27 kg) are at room temperature of 20.0°C.What is the de Broglie wavelength of the helium atoms that are moving at the root-mean-square speed? (h = 6.626 × 10-34 J • s,the Boltzmann constant is 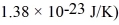
A) 5.22 × 10-11 m
B) 7.38 × 10-11 m
C) 1.04 × 10-10 m
D) 2.82 × 10-10 m
E) 3.99 × 10-10 m
Correct Answer:

Verified
Correct Answer:
Verified
Q9: The wavelength of a ruby laser is
Q10: The energy of an electron state has
Q11: The Bohr radius of the hydrogen atom
Q12: Light excites atomic hydrogen from its lowest
Q15: An electric current through a tungsten filament
Q16: In a double slit experiment,a beam of
Q17: An electron inside a hydrogen atom is
Q18: A hydrogen atom makes a downward transition
Q19: If the accuracy in measuring the position
Q19: An electric current through a tungsten filament