Multiple Choice
An alkali metal atom is in the ground state.The orbital angular momentum equals zero and the spin angular momentum is entirely due to the single valence electron.A magnetic field is applied that splits the ground state energy level into two levels,65 μeV apart.A photon,absorbed by the atom,induces a transition between the two levels.What is the wavelength of the photon? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J • s,Bohr magneton = μB = 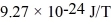 ,
, 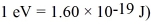
A) 19 mm
B) 25 mm
C) 31 mm
D) 38 mm
E) 41 mm
Correct Answer:

Verified
Correct Answer:
Verified
Q13: The normalized wave function for a hydrogen
Q14: The magnitude of the orbital angular momentum
Q15: If two electrons in an atom have
Q16: The only INVALID electron state and shell
Q17: The binding energy of the hydrogen atom
Q19: What is the electron configuration for ground
Q20: An s state (l = 0)energy level
Q21: If the orbital quantum number is l
Q22: An electron in a hydrogen atom is
Q23: Consider the n = 9 shell.<br>(a)What is