Multiple Choice
The normalized wave function for a hydrogen atom in the 1s state is given by 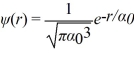 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
A) 2.3 × 10-5
B) 1.2 × 10-5
C) 1.7 × 10-5
D) 4.6 × 10-5
E) 3.5 × 10-5
Correct Answer:

Verified
Correct Answer:
Verified
Q8: A neutral atom has an electron configuration
Q9: An atom has completely filled inner shells
Q10: Model a hydrogen atom as a three-dimensional
Q11: The energy of an electron in the
Q12: How fast must a hydrogen atom be
Q14: The magnitude of the orbital angular momentum
Q15: If two electrons in an atom have
Q16: The only INVALID electron state and shell
Q17: The binding energy of the hydrogen atom
Q18: An alkali metal atom is in the