Multiple Choice
Model a hydrogen atom as a three-dimensional potential well with U0 = 0 in the region 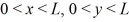 ,and 0 < z < L,and infinite otherwise,with L = 1.0 × 10-10 m.Which of the following is NOT one of the lowest three energy levels of an electron in this model?
,and 0 < z < L,and infinite otherwise,with L = 1.0 × 10-10 m.Which of the following is NOT one of the lowest three energy levels of an electron in this model?
A) 113 eV
B) 226 eV
C) 283 eV
D) 339 eV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: What is the greatest total angular momentum
Q6: An electron initially in a 4p state
Q7: An alkali metal atom is in the
Q8: A neutral atom has an electron configuration
Q9: An atom has completely filled inner shells
Q11: The energy of an electron in the
Q12: How fast must a hydrogen atom be
Q13: The normalized wave function for a hydrogen
Q14: The magnitude of the orbital angular momentum
Q15: If two electrons in an atom have