Multiple Choice
If we double the root-mean-square speed (thermal speed) of the molecules of a gas,then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of 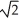
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: A sealed 26- m<sup>3</sup> tank is filled
Q11: The root-mean-square speed (thermal speed)of the molecules
Q12: For a fixed amount of gas,if the
Q14: What is the average kinetic energy of
Q16: A vertical tube that is closed at
Q27: A sample of an ideal gas is
Q33: At 50.0°C,the average translational kinetic energy of
Q34: What is the average translational kinetic energy
Q49: The average molecular kinetic energy of a
Q52: The root-mean-square speed (thermal speed)of the molecules