Short Answer
The figure shows a pV diagram of a gas for a complete cycle.During part bc of the cycle,1190 J of thermal energy through the process of heating flows into a system,and at the same time the system expands against a constant external pressure of as its volume increases from to Calculate the change in internal (thermal)energy of the system during part bc of the cycle.If the change is nonzero,be sure to indicate whether the change is positive or negative. 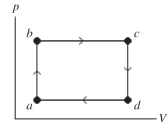
Correct Answer:

Verified
Correct Answer:
Verified
Q13: On a cold day,a piece of metal
Q17: A thermally isolated system is made up
Q19: How much thermal energy must be removed
Q19: If 50 g of lead (of specific
Q20: Some properties of a certain glass
Q22: The gas in a perfectly insulated
Q23: A lab assistant pours 330 g
Q24: A gas expands from an initial volume
Q25: The process shown on the TV graph
Q26: An athlete doing push-ups performs 650 kJ