The Phase Diagram for Carbon Dioxide Is Given Below C,at 1 Atm and Room Temperature
A)solid Carbon Dioxide Is
Multiple Choice
The phase diagram for carbon dioxide is given below. 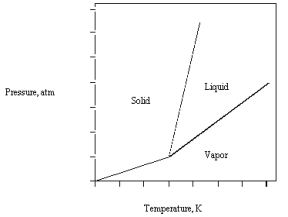
If the triple point is at 5.1 atm and -56 C,at 1 atm and room temperature
A) solid carbon dioxide is the stable phase.
B) liquid carbon dioxide is the stable phase.
C) gaseous carbon dioxide condenses.
D) gaseous carbon dioxide is the stable phase.
E) solid carbon dioxide melts.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Using a mass of 500.0 g of
Q66: Which of the following liquids freeze at
Q84: The following phase diagram is for a
Q85: Consider the diagram below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1441/.jpg" alt="Consider
Q86: The enthalpy of hydration of AgBr
Q87: Why is the vapor pressure of trans-dibromoethene
Q88: The osmotic pressure of 1.00 g
Q89: The phase diagram for CO<sub>2</sub> is
Q92: The vapor pressures of pure carbon disulfide
Q134: Calculate the vapor pressure at25<sup> <span