Multiple Choice
The phase diagram for CO2 is given below.The triple point is at 5.1 atm and 217 K. 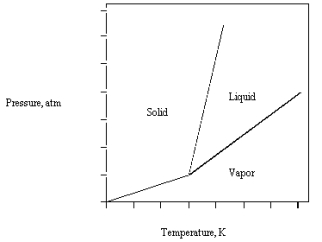
What happens if carbon dioxide at -50 C and 25 atm is suddenly brought to 1 atm?
A) The liquid and solid are in equilibrium.
B) The solid melts.
C) The solid and vapor are in equilibrium.
D) The solid vaporizes.
E) The solid remains stable.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Using a mass of 500.0 g of
Q66: Which of the following liquids freeze at
Q84: The following phase diagram is for a
Q85: Consider the diagram below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1441/.jpg" alt="Consider
Q86: The enthalpy of hydration of AgBr
Q87: Why is the vapor pressure of trans-dibromoethene
Q88: The osmotic pressure of 1.00 g
Q91: The phase diagram for carbon dioxide
Q92: The vapor pressures of pure carbon disulfide
Q134: Calculate the vapor pressure at25<sup> <span