True/False
Use the following to answer questions 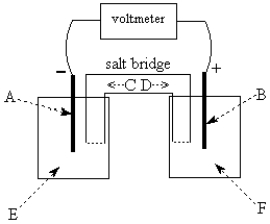
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or lead.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Consider the following cell:<br>Ag(s)|Ag<sup>+</sup>(aq,0.100 M)mAg<sup>+</sup>(aq,0.100 M)|Ag(s)<br>What is
Q66: Which species will reduce Br<sub>2</sub> but not
Q75: How many electrons appear in the
Q76: Which of the following occurs when HCl(aq),Cu(s),and
Q80: Given the half reaction: NO<sub>3</sub><sup>-</sup>(aq) <span
Q82: When equilibrium is reached in an electrochemical
Q83: Consider the following cell: Pt|H<sub>2</sub>(g,1 atm)|H<sup>+</sup>(aq,?
Q84: The standard voltage of the cell
Q156: How long will it take to deposit
Q267: Use the following to answer questions 55-58: