Multiple Choice
Use the following diagram of a cell to answer questions 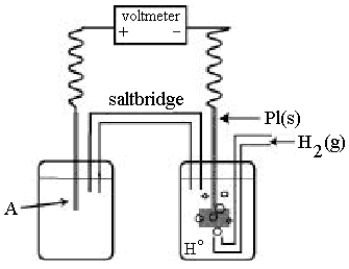
-Using the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .When the voltmeter reading is -0.80 V,which half-reaction occurs in the left-hand cell compartment?
A) Ag(s) Ag+(aq) + e-
B) Ag+(aq) + e- Ag(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q15: What is the value of E
Q42: For the reduction of Cu<sup>2+</sup> by
Q43: In a working electrochemical cell (+ cell
Q50: The standard potential of the Cu<sup>2+</sup>/Cu electrode
Q51: Use the following diagram of a cell
Q113: The half-reaction that occurs at cathode when
Q117: When KI(aq)is electrolyzed at a concentration of
Q176: How many seconds are required to produce
Q183: Of the following metals, which metal would
Q256: Which of the following metals would be