Short Answer
Use the following diagram of a cell to answer questions 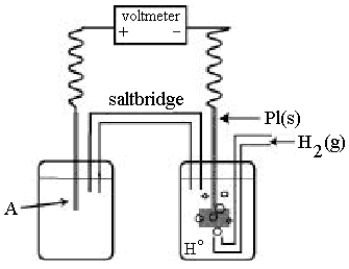
-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,which electrode is negative?
Correct Answer:

Verified
Correct Answer:
Verified
Q15: What is the value of E
Q47: Use the following diagram of a
Q50: The standard potential of the Cu<sup>2+</sup>/Cu electrode
Q53: The standard potential of the Cu<sup>2+</sup>/Cu electrode
Q54: In the determination of iron in vitamins,Fe<sup>2+</sup>
Q55: Consider the following reaction: 2Cu<sup>+</sup>(aq) <span
Q56: Given: Cr(OH)<sub>3</sub>(s) <span class="ql-formula" data-value="\rightarrow"><span class="katex"><span
Q113: The half-reaction that occurs at cathode when
Q176: How many seconds are required to produce
Q256: Which of the following metals would be