Multiple Choice
Consider the molecule below.Determine the molecular geometry at each of the 2 labeled carbons. 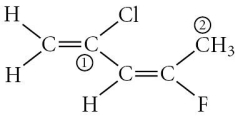
A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2= bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Use the molecular orbital diagram shown to
Q37: Give the number of sigma bonds and
Q38: Give the hybridization for the S in
Q39: Determine the electron geometry (eg)and molecular geometry
Q40: A molecule with a T-shaped molecular geometry
Q42: Determine the electron geometry (eg)and molecular geometry
Q43: Consider the molecule below.Determine the hybridization at
Q44: Determine the electron geometry (eg),molecular geometry (mg),and
Q45: Explain why oil and water do not
Q46: Draw the molecular orbital diagram shown to