Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is most stable. 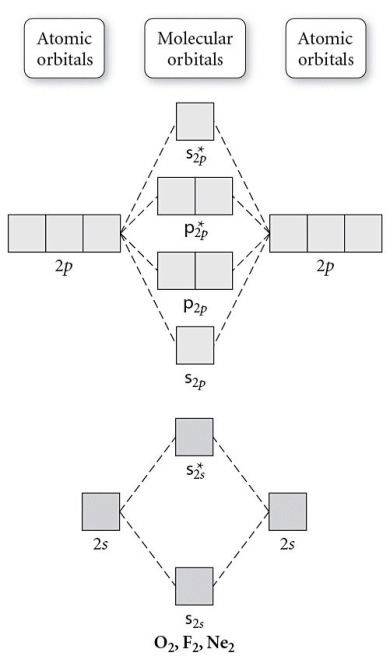
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Give the hybridization for the Br in
Q32: A molecule containing a central atom with
Q33: Determine the electron geometry (eg),molecular geometry (mg),and
Q34: Describe a pi bond.<br>A) side by side
Q35: A molecule,that is sp<sup>3</sup>d hybridized and has
Q37: Give the number of sigma bonds and
Q38: Give the hybridization for the S in
Q39: Determine the electron geometry (eg)and molecular geometry
Q40: A molecule with a T-shaped molecular geometry
Q41: Consider the molecule below.Determine the molecular geometry