Multiple Choice
What is the net ionic equation for the neutralization of hydroiodic acid with aqueous potassium hydroxide?
A) H+(aq) + OH-(aq) H2O( 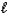 )
)
B) H+(aq) + I-(aq) + K+(aq) + OH-(aq) KI(aq) + H2O( 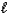 )
)
C) H+(aq) + I-(aq) + K+(aq) + OH-(aq) HI(aq) + KOH(aq)
D) HI(aq) + K+(aq) + OH-(aq) KI(aq) + H2O( 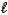 )
)
E) HI(aq) + KOH(aq) KI(aq) + H2O( 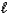 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q4: A(n)_ agent gains electrons in an oxidation-reduction
Q12: Nitric acid is the product of the
Q13: Which of the following is/are classified
Q15: Which species is reduced in the
Q16: The oxidation number of nitrogen is highest
Q20: Which of the following combinations will produce
Q21: Write a balanced net ionic equation
Q22: Which of the following would
Q23: Which of the following reactions best
Q68: Metal oxides react with water to produce