Multiple Choice
Write a balanced net ionic equation for the reaction of aqueous solutions of baking soda (NaHCO3) and acetic acid.
A) HCO3-(aq) + CH3CO2H(aq) CH3CO2-(aq) + H2O( 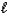 ) + CO2(g)
) + CO2(g)
B) 2 NaHCO3(aq) + CH3CO2H(aq) 2 Na2CO3(aq) + CH4(aq) + 2H2O( 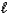 ) + CO2(g)
) + CO2(g)
C) NaHCO3(aq) + H+(aq) H2CO3(s) + Na+(aq)
D) HCO3-(aq) + H+(aq) H2O( 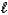 ) + CO2(g)
) + CO2(g)
E) HCO3-(aq) + H+(aq) H2CO3(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q4: A(n)_ agent gains electrons in an oxidation-reduction
Q16: The oxidation number of nitrogen is highest
Q18: What is the net ionic equation
Q20: Which of the following combinations will produce
Q22: Which of the following would
Q23: Which of the following reactions best
Q24: What is the smallest whole number
Q25: Which of the following ions is
Q26: Write a balanced equation for the reaction
Q68: Metal oxides react with water to produce