Multiple Choice
All of the following are oxidation-reduction reactions EXCEPT
A) CaCO3(s) CaO(s) + CO2(g)
B) 2 Na(s) + Br2(g) 2 NaBr(g)
C) Fe(s) + 2 HCl(aq) FeCl2(aq) + H2(g)
D) 2 C(s) + O2(g) 2 CO(g)
E) 2 H2O( 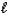 ) 2 H2(g) + O2(g)
) 2 H2(g) + O2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Write a balanced chemical equation for
Q29: Which of the following is a
Q30: If an aqueous solution of _ is
Q30: Give the name of an acidic oxide
Q31: Nitroglycerin decomposes violently according to the
Q33: What are the smallest whole number
Q35: What are the spectator ions in the
Q36: What is the net ionic equation
Q37: Which of the following is a
Q72: Which of the following compounds is a