Multiple Choice
Nitroglycerin decomposes violently according to the balanced chemical equation below.2 C3H5(NO3) 3( 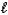 ) 3 N2(g) + 1/2 O2(g) + 6 CO2(g) + 5 H2O(g)
) 3 N2(g) + 1/2 O2(g) + 6 CO2(g) + 5 H2O(g)
Which of the following statements concerning this reaction is/are CORRECT?
1) Two moles of nitroglycerine will produce three moles of nitrogen and five moles of water.
2) Four molecules of nitroglycerine will produced one molecule of oxygen and twelve molecules of carbon dioxide.
3) Six grams of nitroglycerine will produce nine grams of nitrogen and fifteen grams of water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Write a balanced equation for the reaction
Q28: Write a balanced chemical equation for
Q29: Which of the following is a
Q30: If an aqueous solution of _ is
Q30: Give the name of an acidic oxide
Q32: All of the following are oxidation-reduction
Q33: What are the smallest whole number
Q35: What are the spectator ions in the
Q36: What is the net ionic equation
Q72: Which of the following compounds is a