Multiple Choice
The thermochemical equation for the combustion of methanol is shown below.
CH3OH( 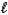 ) + 3/2 O2(g) CO2(g) + 2 H 2O(g)
) + 3/2 O2(g) CO2(g) + 2 H 2O(g)
rH = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g CH3OH?
A) -171 kJ
B) -19.9 kJ
C) -2.38 103 kJ
D) -5.49 103 kJ
E) -1.76 106 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q21: If 35.0 g H<sub>2</sub>O at 22.7
Q22: When 10.0 g KOH is dissolved
Q24: If 46.1 g Cu at 11.6
Q25: Which of the following statements is/are CORRECT?<br>1)A
Q27: The energy stored in a fuel is
Q28: A bomb calorimeter has a heat
Q29: How much heat is liberated at
Q31: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q32: It is relatively easy to change the
Q61: In thermodynamics,a(n)_ is defined as the object,or