Multiple Choice
Calculate rH for the combustion of ammonia,
4 NH3(g) + 7 O2(g) 4 NO2(g) + 6 H2O( 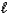 )
)
Using standard molar enthalpies of formation.
A) +30.24 kJ/mol-rxn
B) -206.9 kJ/mol-rxn
C) -298.6 kJ/mol-rxn
D) -1398.8 kJ/mol-rxn
E) -1663.6 kJ/mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: The standard molar enthalpy of formation of
Q26: The thermochemical equation for the combustion
Q27: The energy stored in a fuel is
Q28: A bomb calorimeter has a heat
Q29: How much heat is liberated at
Q32: It is relatively easy to change the
Q33: When 66.0 g of an unknown
Q34: CaO(s)reacts with water to form Ca(OH)<sub>2</sub>(aq).If
Q36: Exactly 212.2 J will raise the temperature
Q44: Many homes are heated using natural gas.The