Multiple Choice
Hydrazine,N2H4,is a liquid used as a rocket fuel.It reacts with oxygen to yield nitrogen gas and water.
N2H4( 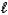 ) + O2(g) N2(g) + 2 H2O(
) + O2(g) N2(g) + 2 H2O( 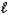 )
)
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
A) -19.4 kJ/mol
B) -25.6 kJ/mol
C) -126 kJ/mol
D) -622 kJ/mol
E) -820.kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Combustion of 7.21 g of liquid
Q38: Determine the heat of evaporation of carbon
Q39: The specific heat capacity of copper
Q40: What quantity,in moles,of oxygen is consumed
Q40: Internal energy and enthalpy are state functions.What
Q41: A 41.3-g piece of nickel (s =
Q43: What is <span class="ql-formula" data-value="\Delta"><span
Q44: Which of the following processes will result
Q46: Calculate the energy in the form
Q47: Determine the enthalpy change for the