Multiple Choice
Determine the heat of evaporation of carbon disulfide,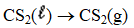
Given the enthalpies of reaction below.
A) -206.1 kJ
B) -27.3 kJ
C) +27.3 kJ
D) +206.1 kJ
E) +1.31 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: When 66.0 g of an unknown
Q34: CaO(s)reacts with water to form Ca(OH)<sub>2</sub>(aq).If
Q36: Exactly 212.2 J will raise the temperature
Q37: Combustion of 7.21 g of liquid
Q39: The specific heat capacity of copper
Q40: What quantity,in moles,of oxygen is consumed
Q41: A 41.3-g piece of nickel (s =
Q42: Hydrazine,N<sub>2</sub>H<sub>4</sub>,is a liquid used as a
Q43: What is <span class="ql-formula" data-value="\Delta"><span
Q44: Many homes are heated using natural gas.The