Multiple Choice
A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.105-g sample of a certain hydrocarbon was burned in this calorimeter,the temperature increased by 2.14 K.Calculate the energy of combustion for 1 g of the hydrocarbon.
A) -5.29 J/g
B) 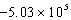 J/g
J/g
C) -0.120 J/g
D) 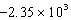 J/g
J/g
E) -0.560 J/g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: The heat required to convert a solid
Q5: Why are you at greater risk
Q6: When 1 mole of Fe<sub>2</sub>O<sub>3</sub>(s)reacts with
Q8: The standard enthalpy change for the
Q10: Iron oxide reacts with aluminum in
Q11: At constant pressure and 25<sup> <span
Q12: Which of the following chemical equations
Q14: The thermochemical equation for the combustion
Q18: One statement of the first law of
Q103: Which of the following is an endothermic