Multiple Choice
When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) according to the following equation,98.8 kJ of energy are absorbed.
Fe2O3(s) + 3 H2(g) 2 Fe(s) + 3 H2O(g)
(A) 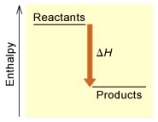
(B) 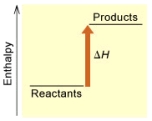
Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
A) endothermic,A
B) endothermic,B
C) exothermic,A
D) exothermic,B
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the following processes is/are endothermic?<br>1)the
Q3: A chemical reaction in a bomb
Q5: Why are you at greater risk
Q8: The standard enthalpy change for the
Q9: A bomb calorimeter has a heat capacity
Q10: Iron oxide reacts with aluminum in
Q11: At constant pressure and 25<sup> <span
Q18: One statement of the first law of
Q43: Which one of the following statements is
Q103: Which of the following is an endothermic