Multiple Choice
Which of the underlined atoms (C1,C2,N,and O) are sp hybridized? 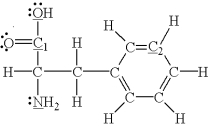
A) C1 and C2
B) C1,C2,N,and O
C) N and O
D) C1,C2,and N
E) none
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the hybridization of the central
Q3: Which molecule will have the following valence
Q5: Diagram 9-1 The molecular orbital diagram
Q9: Diagram 9-1 The molecular orbital diagram
Q10: Diagram 9-1 The molecular orbital diagram
Q11: The electron configuration of a particular
Q12: Diagram 9-1 The molecular orbital diagram
Q13: Diagram 9-1 The molecular orbital diagram
Q19: Refer to Diagram 9-1.Assuming that the molecular
Q63: Refer to Diagram 9-1.According to molecular orbital