Multiple Choice
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 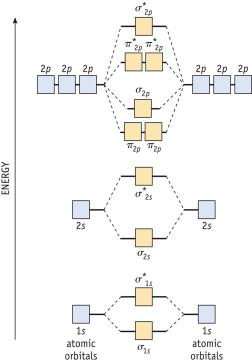
-Refer to Diagram 9-1.Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule,determine which of the following species is paramagnetic.
A) NO+
B) CO
C) CN-
D) OF-
E) NO
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which of the underlined atoms (C<sub>1</sub>,C<sub>2</sub>,N,and O)are
Q9: Diagram 9-1 The molecular orbital diagram
Q10: Diagram 9-1 The molecular orbital diagram
Q11: The electron configuration of a particular
Q13: Diagram 9-1 The molecular orbital diagram
Q15: If 10 orbitals on one atom overlap
Q16: Which of the following hybridized atoms is
Q54: Ammonia reacts with oxygen and water to
Q56: Refer to Diagram 9-1.Consider the molecules B<sub>2</sub>,C<sub>2</sub>,N<sub>2</sub>
Q63: Refer to Diagram 9-1.According to molecular orbital