Multiple Choice
Which process is accompanied by the change in energy known as the lattice enthalpy of RbCl?
A) Rb+(g) + Cl-(g) Rb+(aq) + Cl-(aq)
B) RbCl(s) RbCl(aq,1 m)
C) Rb+(g) + Cl-(g) RbCl(s)
D) Rb(s) + 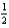 Cl2(g) RbCl(s)
Cl2(g) RbCl(s)
E) Rb(s) + 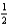 Cl2(g) RbCl(aq,1 m)
Cl2(g) RbCl(aq,1 m)
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Which of the following compounds is not
Q42: Which of the following favor(s)the solubility of
Q73: Which of the following statements concerning osmosis
Q75: For the following gas-aqueous liquid equilibrium
Q76: What mass of Na<sub>2</sub>SO<sub>4</sub> must be
Q77: What is the mole fraction of urea,CO(NH<sub>2</sub>)<sub>2</sub>,<sub>
Q78: A 2.3-g sample of a small protein
Q79: What partial pressure of oxygen gas
Q80: A concentrated hydrochloric acid solution has a
Q82: The Henry's law constant for N<sub>2</sub>